Part Production Approval Process (PPAP)
Part Uniformity
The Part Production Approval Process (PPAP) was generated by the automotive industry, in an effort to ensure that parts approved for automotive applications were of uniform quality and dimension. The automotive supply chain is large and complex, and part uniformity is essential for a quality vehicle. Therefore, the automotive industry created the PPAP standard. The PPAP standard is published by the Automotive Industry Action Group (AIAG), and it provides guidance on how the process works. In practice, no two PPAPs are alike, as a certain amount of negotiation between the supplier and the customer occurs as to what will constitute an acceptable process for a part. Common to all PPAP systems is extensive documentation and measurement of the product, especially at the prototype and initial production run stage including verification and validation. This can include gauge R&R, MSA, MTE evaluations as well as software and process validation. Run at rate testing can also be performed during the PPAP to determine if a manufacturer can produce the required number of pieces per day, with the required uniformity. It is typically used when the number of parts produced will be on a continuous basis, with lot sizes of at least 500 pieces, but has been used for smaller production runs using special sampling plans. PPAP sampling plans are statistical in nature, so tend to work better for larger production lots.
Critical Parameters and Components
As part of the PPAP, several critical factors will be determined for the product – for the product itself or for the components of the product. These are typically called out as critical parameters or critical components. For the product, critical parameters will be called out that will need to be measured and checked prior to shipment to the customer, while for critical components various verification parameters will be called out to be checked before use in the product. As the number of pieces associated with these items is typically very large (think in terms of thousands or millions of units), rather than perform arduous, time-consuming 100% inspection of these items, a smaller sample of these items would be evaluated. This would be referred to as a sampling plan, usually conducted under the guidance of a standard like ISO-2859, MIL-STD105E or ANSI Z1.4 / Z1.9. At a decided-upon lot size, the specified number of pieces is inspected, the result of which is evaluated against the plans pass/fail criteria to determine if the lot is acceptable or rejectable (re-work required), prior to use or shipment. If the part and product measurements indicating the specification requirement are met, then you proceed to the next step in the process. If the parts or products do not meet sampling plan requirements, the parts will be disposed of, as was determined at the PPAP stage. Outright rejection and scrap may be the outcome. More typically, the customer or supplier would be informed, then a more aggressive sampling plan to cull out or re-work the defective items would be instituted. QMS systems functioning under this kind of approval process assume all parts, pieces and processes are tagged as rejected units until such time as the inspection result shows that they are acceptable. Lacking documentation of a product’s measurement results would be a rejected condition.
See Figure 1 of an overview of the PPAP process.
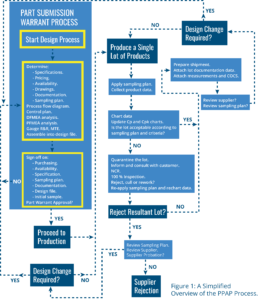
The accepted conditions for parts, processes and product are specifically documented, at the Part Submission Warrant stage of the process. It is at this stage, the specification, design, FMEAs (either design or process), prototyping and production sample phase, that all of the associated parameters and specifications are decided, documented and approved. These typically emerge as control documents and process plans. Note that the warrant is for the processes and the parts. Reject conditions on the production process aspect of the production item can also exist. Flow charts are typically used to document process plans. Specifications and documentation are typically associated with the parts. Process charts are usually associated with process quality. Various parameters can be charted, like run charts for critical component measurements, CP and CPk charts as measures of process capability and stability. CP is a measure of process parameter stability, where CPk is a measure of how well the process is centered on its specified range. The difference can be visualized as throwing darts at a dartboard. If the centre of the dartboard is seen as the target, then if the darts thrown all land on the triple 20, the dart-throwing process will have a good CP; you are hitting the target consistently (the triple 20), but not a good CPk, as the darts are not landing in and around the desired target consistently (the darts are not centered around or hitting the bullseye). Run charts, Cp and CPk charts can be used as measures of process quality and stability.
The Feedback Process
There is a feedback process associated with PPAP. During the part warrant stage of the PPAP a typical stipulation is put in place that changes to the part or process will not occur without express written consent of both parties to the PPAP warrant. If such changes become necessary or desired due to ordinary conditions (desire to increase profits by using alternate vendors) or extraordinary conditions (pre-approved parts become unavailable), change notifications are issued. The results of a change notification can be varied, both parties may accept the change unconditionally, one party may refuse any changes at all, or it may be decided to re-run the part warranty process completely. Regardless of the outcome, it is necessary for both parties to be informed of any proposed changes to the product, and to agree to the changes, with formal documentation.
Partnership of PPAP and Medical
The tie-in to medical devices with PPAP occurred several years ago. Major automobile manufacturers developed this process to ensure that automotive parts suppliers could produce highly-uniform, high-quality parts in desired quantities. Several parts suppliers to this sector participated in the procedure. As a result, these parts suppliers became familiar with these techniques in making high-quality parts with the documentation trail associated with the PPAP process. Another manufacturing field that also requires highly-uniform, high-quality parts with a stable supply chain is the medical device field. As a result, the AIAG offered ISO 13485 (Medical Device Quality System Standard) classes to auto parts suppliers that wanted to diversify into the medical device field. A key part of the ISO 13485 standard is the medical device file. A PPAP product warrant will typically satisfy the requirements for a medical device file. Hence companies familiar with making products under a PPAP warrant, find that meeting the requirements of a medical device file to be familiar territory, accommodated by their already existing systems.
Tools and Documents
There are many associated PPAP tools and documents. Some typical ones are:
- PFMEA – Process Failure Mode Effect Analysis
- DFMEA – Design Failure Mode Effect Analysis
- SPC – Statistical Process Control
- MSA – Measurement System Analysis
- GR&R – Gauge Repeatability and Reproducibility
- APQP – Advanced Product Quality Planning
- PSW – Part Submission Warrant
- MTE – Measurement and Test equipment Evaluation
- APQP – Advanced Product Quality Planning
- FAI – First Article Inspection




